Abstract
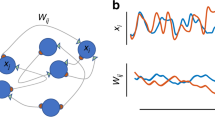
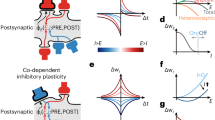
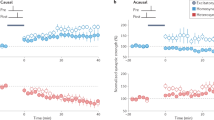
Recent experiments demonstrate substantial volatility of excitatory connectivity in the absence of any learning. This challenges the hypothesis that stable synaptic connections are necessary for long-term maintenance of acquired information. Here we measure ongoing synaptic volatility and use theoretical modeling to study its consequences on cortical dynamics. We show that in the balanced cortex, patterns of neural activity are primarily determined by inhibitory connectivity, despite the fact that most synapses and neurons are excitatory. Similarly, we show that the inhibitory network is more effective in storing memory patterns than the excitatory one. As a result, network activity is robust to ongoing volatility of excitatory synapses, as long as this volatility does not disrupt the balance between excitation and inhibition. We thus hypothesize that inhibitory connectivity, rather than excitatory, controls the maintenance and loss of information over long periods of time in the volatile cortex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The dataset analyzed in the current study is available in http://bio.huji.ac.il/yonatanlab/spines/.
Change history
01 November 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Lai, C. S., Franke, T. F. & Gan, W. B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483, 87–91 (2012).
Moczulska, K. E. et al. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc. Natl. Acad. Sci. USA 110, 18315–18320 (2013).
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009).
Yang, G., Pan, F. & Gan, W. B. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009).
Hayashi-Takagi, A. et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338 (2015).
Caroni, P., Donato, F. & Muller, D. Structural plasticity upon learning: regulation and functions. Nat. Rev. Neurosci. 13, 478–490 (2012).
Zuo, Y., Lin, A., Chang, P. & Gan, W. B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005).
Holtmaat, A. J. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
Loewenstein, Y., Yanover, U. & Rumpel, S. Predicting the dynamics of network connectivity in the neocortex. J. Neurosci. 35, 12535–12544 (2015).
Chambers, A. R. & Rumpel, S. A stable brain from unstable components: Emerging concepts and implications for neural computation. Neuroscience 357, 172–184 (2017).
Mongillo, G., Rumpel, S. & Loewenstein, Y. Intrinsic volatility of synaptic connections–a challenge to the synaptic trace theory of memory. Curr. Opin. Neurobiol. 46, 7–13 (2017).
Holtmaat, A. & Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658 (2009).
Kasai, H., Fukuda, M., Watanabe, S., Hayashi-Takagi, A. & Noguchi, J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129 (2010).
Knott, G. W., Holtmaat, A., Wilbrecht, L., Welker, E. & Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 9, 1117–1124 (2006).
Loewenstein, Y., Kuras, A. & Rumpel, S. Multiplicative dynamics underlie the emergence of the log-normal distribution of spine sizes in the neocortex in vivo. J. Neurosci. 31, 9481–9488 (2011).
DeFelipe, J. & Jones, E. G. in Handbook of Brain Microcircuits (eds. Shepherd, G. M. & Grillner, S.). Ch. 1,5–14 (Oxford University Press, 2010).
Gentet, L. J., Avermann, M., Matyas, F., Staiger, J. F. & Petersen, C. C. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65, 422–435 (2010).
Karnani, M. M., Agetsuma, M. & Yuste, R. A blanket of inhibition: functional inferences from dense inhibitory connectivity. Curr. Opin. Neurobiol. 26, 96–102 (2014).
Bhatt, D. H., Zhang, S. & Gan, W. B. Dendritic spine dynamics. Annu. Rev. Physiol. 71, 261–282 (2009).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Avermann, M., Tomm, C., Mateo, C., Gerstner, W. & Petersen, C. C. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J. Neurophysiol. 107, 3116–3134 (2012).
Buzsáki, G. & Mizuseki, K. The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 15, 264–278 (2014).
Renart, A. et al. The asynchronous state in cortical circuits. Science 327, 587–590 (2010).
van Vreeswijk, C. & Sompolinsky, H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726 (1996).
Roxin, A., Brunel, N., Hansel, D., Mongillo, G. & van Vreeswijk, C. On the distribution of firing rates in networks of cortical neurons. J. Neurosci. 31, 16217–16226 (2011).
Hendin, O., Horn, D. & Tsodyks, M. V. The role of inhibition in an associative memory model of the olfactory bulb. J. Comput. Neurosci. 4, 173–182 (1997).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88 (2005).
Landau, I. D., Egger, R., Dercksen, V. J., Oberlaender, M. & Sompolinsky, H. The impact of structural heterogeneity on excitation-inhibition balance in cortical networks. Neuron 92, 1106–1121 (2016).
Froemke, R. C., Merzenich, M. M. & Schreiner, C. E. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007).
Rubinski, A. & Ziv, N. E. Remodeling and tenacity of inhibitory synapses: relationships with network activity and neighboring excitatory synapses. PLoS Comput. Biol. 11, e1004632 (2015).
Denève, S. & Machens, C. K. Efficient codes and balanced networks. Nat. Neurosci. 19, 375–382 (2016).
Hennequin, G., Vogels, T. P. & Gerstner, W. Optimal control of transient dynamics in balanced networks supports generation of complex movements. Neuron 82, 1394–1406 (2014).
Griffen, T. C. & Maffei, A. GABAergic synapses: their plasticity and role in sensory cortex. Front. Cell. Neurosci. 8, 91 (2014).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Doron, G., von Heimendahl, M., Schlattmann, P., Houweling, A. R. & Brecht, M. Spiking irregularity and frequency modulate the behavioral report of single-neuron stimulation. Neuron 81, 653–663 (2014).
Liberti, W. A. et al. Unstable neurons underlie a stable learned behavior. Nat. Neurosci. 19, 1665–1671 (2016).
Chen, J. L. et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373 (2012).
van Versendaal, D. et al. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383 (2012).
Hensch, T. K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Huang, Z. J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Levelt, C. N. & Hübener, M. Critical-period plasticity in the visual cortex. Annu. Rev. Neurosci. 35, 309–330 (2012).
Letzkus, J. J., Wolff, S. B. & Lüthi, A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276 (2015).
Driscoll, L. N., Pettit, N. L., Minderer, M., Chettih, S. N. & Harvey, C. D. Dynamic reorganization of neuronal activity patterns in parietal cortex. Cell 170, 986–999.e16 (2017).
Maass, W. Searching for principles of brain computation. Curr. Opin. Behav. Sci. 11, 81–92 (2016).
Gaiarsa, J. L., Caillard, O. & Ben-Ari, Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 25, 564–570 (2002).
Kullmann, D. M., Moreau, A. W., Bakiri, Y. & Nicholson, E. Plasticity of inhibition. Neuron 75, 951–962 (2012).
Woodin, M. A., Ganguly, K. & Poo, M. M. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron 39, 807–820 (2003).
Donato, F., Rompani, S. B. & Caroni, P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013).
Vogels, T. P., Sprekeler, H., Zenke, F., Clopath, C. & Gerstner, W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573 (2011).
Luz, Y. & Shamir, M. Balancing feed-forward excitation and inhibition via Hebbian inhibitory synaptic plasticity. PLoS Comput. Biol. 8, e1002334 (2012).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Grutzendler, J., Kasthuri, N. & Gan, W. B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Trachtenberg, J. T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Keck, T. et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 11, 1162–1167 (2008).
Kasthuri, N. et al. Saturated reconstruction of a volume of neocortex. Cell 162, 648–661 (2015).
Markram, H. et al. Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492 (2015).
Renart, A., Brunel, N. & Wang, X. Computational Neuroscience: A Comprehensive Approach. (CRC Press, Boca Raton, FL, USA,, 2003). Chapter 15.
Amit, D. J., Gutfreund, H. & Sompolinsky, H. Spin-glass models of neural networks. Phys. Rev. A. Gen. Phys. 32, 1007–1018 (1985).
Acknowledgements
We thank L. Abbott and D. Hansel for their careful reading of our manuscript and their insightful comments. This work was performed in the framework of the the France-Israel Center for Neural Computation and was supported by the Israel Science Foundation (Grant No. 757/16, Y.L.), the DFG (CRC 1080, Y.L. and S.R.), the Gatsby Charitable Foundation (Y.L.), and by ANR (14-NEUC-0001-01 and 13-BSV4-0014-02, G.M.).
Author information
Authors and Affiliations
Contributions
S.R. performed the experiments, G.M., S.R. and Y.L. analyzed the data, G.M. and Y.L. developed the theory, G.M. performed the numerical simulations, G.M., S.R. and Y.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Sensitivity of the firing rate of neurons to the excitatory and inhibitory inputs.
(a), The firing rate of the excitatory neuron of Fig. 4a in response to its excitatory input, measured in units of standard deviation relative to its mean. Line corresponds to average rate and shaded area denote mean ± 2 standard deviations. (b), same as in a for the inhibitory input. Note that the firing rate of the neuron is far more sensitive to its inhibitory input than to its excitatory input. (c), and (d), same as (a), and (b), for the inhibitory neuron in Fig. 4b.
Supplementary Figure 2 Dependence of the effect of rewiring on the distributions of firing rates.
The circles denote the correlation coefficients of the vectors of the firing rates of excitatory (blue) and inhibitory (red), as in Fig. 3, before and after the rewiring of the different synaptic types (from left to right: Top, E→E, E→I; Bottom, I→E, I→I), as a function of the relative averaged squared firing rate of the excitatory neurons. Each circle corresponds to a different value of pair of external inputs. Black diamonds correspond to cortical parameters (Fig. 3). The correlation coefficients and firing rates were computed using the mean field approximation.
Supplementary Figure 3 The effect of different levels of heterogeneous addition of E→E connections to targeted neurons.
Same as in Fig. 7d, the autocorrelogram of the vectors of firing rates of the excitatory ((a), blue) and inhibitory ((b), red) neurons as a function of the fraction of excitatory neurons that were targeted. Dotted line denotes a ΔC = 10% increase in the number of connections (probability of input from previously-unconnected excitatory neurons is 0.025); solid line denoted a ΔC = 20% increase in the number of connections (probability of new connections is 0.05, same as Fig. 7d); Dashed line denotes a ΔC = 40% increase (probability 0.1).
Supplementary information
Rights and permissions
About this article
Cite this article
Mongillo, G., Rumpel, S. & Loewenstein, Y. Inhibitory connectivity defines the realm of excitatory plasticity. Nat Neurosci 21, 1463–1470 (2018). https://doi.org/10.1038/s41593-018-0226-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0226-x
This article is cited by
-
Distributing task-related neural activity across a cortical network through task-independent connections
Nature Communications (2023)
-
Prefrontal parvalbumin interneurons deficits mediate early emotional dysfunction in Alzheimer’s disease
Neuropsychopharmacology (2023)
-
Inhibitory neurons control the consolidation of neural assemblies via adaptation to selective stimuli
Scientific Reports (2023)
-
The plasticitome of cortical interneurons
Nature Reviews Neuroscience (2023)
-
Dynamic branching in a neural network model for probabilistic prediction of sequences
Journal of Computational Neuroscience (2022)